U.S. Food and Drug Administration Approves Humulin® R U-500 KwikPen®
"People with diabetes and severe insulin resistance who have become poorly responsive to the effects of insulin may require much higher insulin doses - more than 200 units per day - to help keep their blood sugar levels on target," said
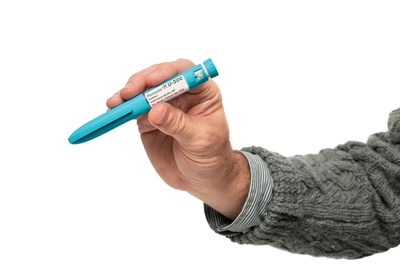
Until now, Humulin R U-500 was only available in a vial, administered with either a U-100 insulin syringe or a volumetric (tuberculin) syringe that requires conversion to respective syringe "unit markings" or volume markings. Although each U-500 KwikPen holds 1500 units of insulin (the amount in five U-100 insulin pens), it is the same size as other KwikPens and dials in five-unit increments. The U-500 KwikPen has a unique aqua-colored pen body to clearly differentiate it from other insulin pens. Healthcare professionals can click here to see the U-500 KwikPen in action.
Humulin R U-500 is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Humulin R U-500 or any of its additives or components. Hypoglycemia (low blood sugar) is the most common side effect associated with
all insulins, including Humulin R U-500. See the Important Safety Information at the end of this press release, Prescribing Information and Patient Information.
The Humulin R U-500 Savings Card Program offers eligible, commercially insured patients the opportunity to pay as little as
"The U-500 KwikPen is another example of Lilly's ongoing commitment to support people living with diabetes and our leadership in the treatment of diabetes," said
For more information about Humulin R U-500 KwikPen, healthcare professionals can visit www.humulinhcp.com.
Important Safety Information for Humulin R U-500
- Contraindications
Humulin R U-500 is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Humulin R U‑500 or any of its excipients.
- Warnings and Precautions
Dosing Errors: Extreme caution must be observed in measuring the dose of Humulin R U‑500 because inadvertent overdose may result in serious adverse reaction or life-threatening hypoglycemia.
Medication errors associated with the Humulin R U‑500 vial have occurred and resulted in patients experiencing hyperglycemia, hypoglycemia, or death.
Dispensing
- Instruct patients to always inspect insulin vials to confirm that the correct insulin is dispensed including the correct brand and concentration.
- The Humulin R U-500 vial, which contains 20 mL, has a band of diagonal brown stripes. "U-500" is also highlighted in red on the Humulin R U-500 vial label.
Prescribing
- When using a U-100 insulin syringe or tuberculin syringe, express the prescribed dose of Humulin R U‑500 in units of insulin along with the appropriate corresponding markings on the syringe the patient is using.
Administration
- Instruct patients to always check the insulin label before each injection.
- A majority of the medication errors with Humulin R U-500 vial occurred due to dosing confusion when the dose was prescribed in units or volume corresponding to a U-100 syringe or tuberculin syringe markings, respectively, or the prescribed dose was administered without recognizing that the markings on the syringe used do not directly correspond to the U-500 dose. Instructions for use should always be read and followed before use.
- Instruct the patient to inform hospital or emergency department staff of the dose of Humulin R U-500 prescribed.
- A conversion chart should always be used when administering doses from the Humulin R U-500 vial with U-100 insulin syringes or 1 mL tuberculin syringes.
If using the Humulin R U‑500 KwikPen, patients should be counseled to dial and dose the prescribed number of units of insulin (NO dose conversion is required).
DO NOT transfer Humulin R U‑500 from the Humulin R U‑500 KwikPen into a syringe for administration. Overdose and severe hypoglycemia can occur.
Patients Should Never Share KwikPens, Needles, or Syringes with Other People, even if the needle is changed. Sharing poses a risk for transmission of blood-borne pathogens.
Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in insulin, manufacturer, type, or method of administration should be made cautiously and only under medical supervision and the frequency of blood glucose monitoring should be increased.
Hypoglycemia: Hypoglycemia is the most common adverse reaction associated with insulin, including Humulin R U‑500. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Severe hypoglycemia may develop as long as 18 to 24 hours after an injection of Humulin R U-500. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important, such as driving or operating other machinery.
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual.
Early warning symptoms of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system, or in patients who experience recurrent hypoglycemia.
The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulation. As with all insulin preparations, the glucose lowering effect time course of Humulin R U-500 may vary in different individuals or at different times in the same individual and depends on many conditions.
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
Hypersensitivity and Allergic Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including Humulin R U-500. If hypersensitivity reactions occur, discontinue Humulin R U‑500; treat per standard of care and monitor until symptoms and signs resolve.
Hypokalemia: Insulin use can lead to hypokalemia that left untreated may cause respiratory paralysis, ventricular arrhythmia, and death. Use caution in patients who may be at risk for hypokalemia (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Thiazolidinediones (TZDs), which are PPAR-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Observe patients for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
- Adverse Reactions include hypoglycemia, allergic reactions, lipodystrophy, injection site reactions, weight gain, peripheral edema, and immunogenicity.
- Drug Interactions: Some medications may alter glucose metabolism and may necessitate insulin dose adjustment. Signs of hypoglycemia may be reduced or absent in patients taking antiadrenergic drugs. Particularly close monitoring may be required.
- Use in Specific Populations
Pregnancy Category B: While there are no adequate and well-controlled studies in pregnant women, evidence from published literature suggests that good glycemic control in patients with diabetes during pregnancy provides significant maternal and fetal benefits.
Pediatric Use: There are no well-controlled studies of use of Humulin R U-500 in children. Standard precautions as applied to use of Humulin R U-500 in adults are appropriate for use in children.
Geriatric Use: There are no well-controlled studies of use of Humulin R U-500 in geriatric patients. In elderly patients with diabetes, the initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemia.
Renal or Hepatic Impairment: Frequent glucose monitoring and insulin dose reduction may be required in patients with renal or hepatic impairment.
- Dosage and Administration
Prescribe Humulin R U-500 ONLY to patients who require more than 200 units of insulin per day.
Adhere to administration instructions to reduce the risk of dosing errors.
Individualize dose of Humulin R U-500 based on metabolic needs, blood glucose monitoring results, and glycemic control goal.
Administer Humulin R U‑500 subcutaneously two or three times daily approximately 30 minutes before a meal. Rotate injection sites to reduce the risk of lipodystrophy.
Do NOT mix Humulin R U-500 with other insulins.
Do NOT administer Humulin R U‑500 intravenously or intramuscularly.
Do NOT perform dose conversion when using the Humulin R U-500 KwikPen. The dose window of the KwikPen shows the number of units of Humulin R U‑500 to be injected and NO dose conversion is required.
Do NOT transfer Humulin R U‑500 from the KwikPen into a syringe.
CONVERT the prescribed dose of Humulin R U-500 into a "unit" or "volume" mark when using the vial and a U-100 or a tuberculin syringe device to deliver Humulin R U-500.
- Storage
Protect from heat and light. Do not freeze. Do not use Humulin R U-500 after the expiration date stamped on the label.
Humulin R U-500 Vials: Unopened vials of Humulin R U-500 should be kept in a refrigerator. Opened (in-use) vials of Humulin R U-500 should be kept in the refrigerator or at room temperature and used within 40 days of opening. Throw away any opened vial after 40 days of use, even if there is insulin left in the vial.
Humulin R U-500 KwikPen: Unopened Humulin R U-500 KwikPens should be kept in a refrigerator. Opened (in-use) Humulin R U-500 KwikPens should be kept at room temperature and used within 28 days of opening. Do not refrigerate opened KwikPens. Throw away any opened KwikPen after 28 days of use, even if there is insulin left in the pen.
HI U500 HCP ISI 04JAN2016
About Lilly Patient Assistance Programs
Lilly offers Patient Assistance programs to help people who may not be able to afford their medications. If a patient financially qualifies and meets program criteria, they may be eligible to receive free product for up to one year. For additional information, visit www.LillyCares.com.
About Diabetes
Approximately 29 million Americans1 and an estimated 415 million people worldwide have type 1 and type 2 diabetes.2 Type 2 diabetes is the most common type, accounting for an estimated 90 to 95 percent of all diabetes cases.1 Diabetes is a chronic disease that occurs when the body either does not properly produce, or use, the hormone insulin.1
About Lilly Diabetes
Lilly has been a global leader in diabetes care since 1923, when we introduced the world's first commercial insulin. Today we work to meet the diverse needs of people with diabetes through research and collaboration, a broad and growing product portfolio and a continued commitment to providing real solutions—from medicines to support programs and more—to make lives better. For more information, visit www.lillydiabetes.com.
About
Lilly is a global healthcare leader that unites caring with discovery to make life better for people around the world. We were founded more than a century ago by a man committed to creating high-quality medicines that meet real needs, and today we remain true to that mission in all our work. Across the globe, Lilly employees work to discover and bring life-changing medicines to those who need them, improve the understanding and management of disease, and give back to communities through philanthropy and volunteerism. To learn more about Lilly, please visit us at www.lilly.com and newsroom.lilly.com/social-channels.
P-LLY
This press release contains forward-looking statements about Humulin R U-500 KwikPen (500 units/mL) for use in the treatment of diabetes. It reflects Lilly's current beliefs; however, as with any such undertaking, there are substantial risks and uncertainties in the process of drug development and commercialization. There is no guarantee that future study results and patient experience will be consistent with study findings to date or that Humulin R U-500 KwikPen will be commercially successful. For further discussion of these and other risks and uncertainties, please see Lilly's latest Forms 10-Q and 10-K filed with the
Humulin® R U-500 and Humulin® R U-500 KwikPen® are registered trademarks owned or licensed by
1.
2.
PP-HM-US-0285 01/2016 ©Lilly
Refer to: Julie Williams, +1-317-627-4056, williamsju@lilly.com


Logo - http://photos.prnewswire.com/prnh/20160115/322609LOGO
Photo - http://photos.prnewswire.com/prnh/20160115/322610
Logo - http://photos.prnewswire.com/prnh/20031219/LLYLOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/us-food-and-drug-administration-approves-humulin-r-u-500-kwikpen-300207789.html
SOURCE
News Provided by Acquire Media
