An Open Letter From Eli Lilly and Company Regarding Certain Practices Related to Mounjaro® and Zepbound®
Lilly is committed to making life better for people living with diabetes and obesity through developing medicines that change the way healthcare providers can treat these diseases. The development and approvals of Mounjaro® and Zepbound® demonstrate our continued commitment to this mission. We are guided every day by our founder Colonel Eli Lilly’s saying when, over a hundred years ago, he affixed his signature as the logo to our medicines: “If it bears a red Lilly, it’s right.”
For those reasons and for the safety of the patients we serve, we wish to respond to certain practices we are seeing from others related to our tirzepatide medicines—Mounjaro® and Zepbound®—to ensure they are prescribed, obtained, and used safely and appropriately.
Please see below for information on each of the following topics:
- Inappropriate Use - Cosmetic Use
- Inappropriate Use - People Under 18
- Mounjaro® and Zepbound® Are The Only FDA-Approved Tirzepatide Medications
- Unsafe Online and Social Media Ads
- Illegal Online Pharmacies
- “Research Purposes” Products
- Misleading Advertisements
- Always Consult Your Healthcare Provider
- Non-Lilly Tirzepatide Can Put People At Risk
- Fake Products
- Unsafe Compounded Products
- Identifying Genuine Lilly Products
Lilly Stands Against the Use of its Medicines for Cosmetic Weight Loss.
Mounjaro® and Zepbound® are indicated for the treatment of serious diseases; they are not approved for – and should not be used for – cosmetic weight loss.
- Lilly does not promote or encourage use of Mounjaro®, Zepbound®, or any Lilly medicine outside of its FDA-approved indication.
- Mounjaro® is an injectable prescription medicine for adults with type 2 diabetes used along with diet and exercise to improve blood sugar (glucose).
- Zepbound® is an injectable prescription medicine that, used along with a reduced-calorie diet and increased physical activity, may help adults with obesity, or with excess weight (overweight) who also have weight-related medical problems, lose weight and keep it off.
- Mounjaro® and Zepbound® should be used only when prescribed by a licensed healthcare professional. Patients should consult with their healthcare providers to determine whether Mounjaro® or Zepbound® is right for them. The labels for Mounjaro® and Zepbound® include a Boxed Warning regarding thyroid C-cell tumors. Mounjaro® and Zepbound® have not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and are therefore not recommended in these patients. Mounjaro® and Zepbound® have not been studied in patients with a prior history of pancreatitis, and it is unknown if patients with a history of pancreatitis are at higher risk for development of pancreatitis on Mounjaro® or Zepbound®. See Important Safety Information and links to the full Prescribing Information and Medication Guide that accompany Mounjaro® and Zepbound®
- If a healthcare provider has questions about the use of Mounjaro® and Zepbound®, they are encouraged to chat directly with a Lilly Medical Professional here or contact Lilly at 1-800-LillyRx (1-800-545-5979).
Mounjaro® and Zepbound® are Not Approved for Use in Patients Under the Age of 18.
FDA has approved Mounjaro® and Zepbound® only for use in people 18 years of age and older.
- Lilly does not promote or encourage the off-label use of Mounjaro® or Zepbound® for anyone, including by persons under the age of 18.
- Social media posts, videos, and ads promoting use of Mounjaro®and Zepbound® in people under 18 are inappropriate and may expose people to significant risks because the safety and efficacy of Mounjaro® and Zepbound® have not been established in people under the age of 18.
Lilly’s Mounjaro® and Zepbound® Are The Only FDA-Approved Tirzepatide Medicines.
Lilly is the only lawful supplier of FDA-approved tirzepatide medicines—Mounjaro® and Zepbound®—and does not provide tirzepatide (the active ingredient in Mounjaro® and Zepbound®) to compounding pharmacies, med-spas, wellness centers, online retailers, or other manufacturers. Lilly does not know where compounding pharmacies or other sellers are obtaining the tirzepatide active ingredient they are selling.
- Lilly will continue to pursue legal remedies against those who falsely claim their products are Mounjaro®, Zepbound®, or “FDA-approved” tirzepatide, including certain med-spas, wellness centers, online retailers, and compounding pharmacies.
- Lilly strongly supports state and federal regulators and law enforcement taking action against those who put patients at risk by selling unsafe or fake products claiming to be tirzepatide.
Unsafe Online Posts, Videos, and Ads Put People At Risk.
Lilly is deeply concerned about the proliferation of online sales and posts on social media involving counterfeit, fake, compounded, and any other unsafe or untested versions of what they say is tirzepatide. Always remember:
- Lilly never sells genuine Mounjaro® or Zepbound® on social media. Any Mounjaro® or Zepbound® offered for purchase on social media is unlawful. These products are either fake or being “resold” by an individual who obtained them through some other means. Both practices put people at risk.
- Illegal online pharmacies “sell substandard and falsified versions” of incretin medications and put people at risk. According to the National Association of Boards of Pharmacy (NABP), “illegal actors are taking advantage of high demand and short supply [of incretin medications] in order to sell substandard and falsified versions of these products to patients around the world.” NABP “has identified thousands of websites that promote the illegal sale of GLP-1 agonists,” including fake or unsafe versions of Mounjaro®, Zepbound®, and “tirzepatide” products. Their “unlawful actions put patients at risk.”
- People should never put products labeled “research purposes only” or “not for human consumption” into their bodies. Lilly is concerned by posts, videos, and ads that promote people taking tirzepatide labeled “research purposes only” and “not for human consumption” for weight loss. Those markings indicate that the products have not been purified to pharmaceutical-grade levels and are not appropriate for human use. Consumers should not use these products—they were not made by, studied by, or sold by Lilly, and may expose people to significant risks.
- Social media is not a replacement for a healthcare professional. Posts, videos, and ads that claim to offer tirzepatide products without a prescription are improper and may expose people to significant risks. FDA has determined that incretins—including Mounjaro®and Zepbound®—require a prescription and must be used under the supervision of a healthcare provider.
Non-Lilly Tirzepatide Can Put People At Risk.
Any products marketed simply as “tirzepatide” (as opposed to Mounjaro® and Zepbound®) were not made by, studied by, or sold by Lilly and are not FDA-approved. Be aware that when you purchase products that are not FDA-approved medicines or obtain medicines from an unverified source or without a prescription from a licensed healthcare provider, you may be purchasing fake, counterfeit, or otherwise unsafe products.
- Fake products designed to look like Lilly’s medicines put people at risk. Lilly has identified fake or counterfeit products that are advertised or designed to look like Lilly’s genuine FDA-approved Mounjaro®and Zepbound® These products are often advertised and sold online, through social media, or at certain med-spas. They are never safe to use.
- Fake or counterfeit products are not inspected by regulatory authorities and may be manufactured in unsanitary and unsafe conditions. They may contain no medicine, the wrong medicine, incorrect dosages, or multiple medicines mixed together, which could result in serious harm.
- FDA recognizes that compounded drugs pose a higher risk than FDA-approved medicines. Although compounding is permitted in limited circumstances for individual patient needs, people should understand that compounded drugs are never FDA-approved.
- FDA has expressly stated “compounded drugs pose a higher risk to patients than FDA-approved drugs,” and that the “unnecessary use of compounded drugs exposes patients to potentially serious health risks.”
- FDA has also “observed troubling conditions” during its inspections of some compounding facilities. That should raise serious concerns, because according to FDA, “poor compounding practices can result in serious drug quality problems, such as contamination,” which “can lead to serious patient injury.”
- Compounded versions of tirzepatide can put people at risk. Sterility is a critical safety concern, given that Mounjaro®and Zepbound® are administered via under-the-skin injection.
- Lilly has discovered compounded drugs advertised as tirzepatide with safety, sterility, and efficacy problems. Some have contained bacteria, high impurity levels, different colors (pink, instead of colorless), or a completely different chemical structure than Lilly’s FDA-approved medicines. In at least one instance, the product was nothing more than sugar alcohol.
- Certain online pharmacies are now advertising compounded pill, under the tongue, nasal spray, and other oral versions of “tirzepatide.” It is important to understand that FDA has only approved administration of tirzepatide via under-the-skin injection. No regulator has evaluated the safety or effectiveness of any oral or nasal administration of “tirzepatide.”
- On May 21, 2024, the Australian government announced a complete ban on compounded tirzepatide and other compounded diabetes and weight loss medicines due to the “increasing reports of patients coming to harm from [incretin medications] including the hospitalization of a patient in Australia due to a serious adverse event.”
- Mark Butler, Australia’s Minister for Health, stated: “You only have to look to the recent reports of individual impacted by large-scale compounding to reali[z]e the dangers posed. This action will protect Australians from harm and save lives.”
- Online ads may be inaccurate or misleading. It’s important to understand both the risks and benefits of a medication before taking it. That’s why anyone who promotes the benefits of a prescription medicine must also disclose the risks posed by the product.
- Some online sellers of compounded tirzepatide promote the potential health benefits of their products (for example, stating they will lead to easy weight management) without disclosing any potential side effects or safety risks of their products. These same online sellers may also be making claims that their non-injection (e.g., oral, under the tongue, nasal spray) tirzepatide products are effective without any scientific support or testing to support those claims.
- Online sellers of compounded tirzepatide also sometimes claim that their “tirzepatide” is manufactured in FDA-approved facilities. However, there is no such thing as an FDA-approved manufacturing facility, and FDA regulations expressly prohibit anyone from making that claim precisely because it is “misleading and constitutes misbranding.”
- Help stop illegal sales by reporting suspected fake or unsafe “tirzepatide” products. If you are concerned that you may have received or used counterfeit, fake, or any otherwise unsafe versions of tirzepatide, you should contact your healthcare provider or seek immediate medical attention. You should also report any such products to local law enforcement or your state’s Board of Pharmacy.
- Lilly also encourages anyone who believes they received or used counterfeit, fake, or any otherwise unsafe version of tirzepatide to contact the Lilly Answers Center (TLAC) at 1-800-LillyRx (1-800-545-5979). We can help direct you to a safe supply of Mounjaro®or Zepbound®, ensure you are aware of our savings card program, and assist you with reporting adverse events or other issues related to your receipt or use of counterfeit, fake, or any otherwise unsafe versions of tirzepatide.
Identifying Genuine Lilly Products.
Non-Lilly tirzepatide products may look very similar to genuine Lilly products. Knowing what an authentic Mounjaro® or Zepbound® pen and its packaging look like may help you avoid purchasing or using counterfeit medications.
Below are photos of authentic Mounjaro® and Zepbound®:
Mounjaro®
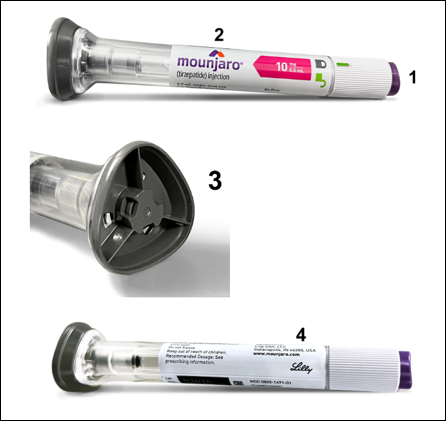 |
|
Zepbound®
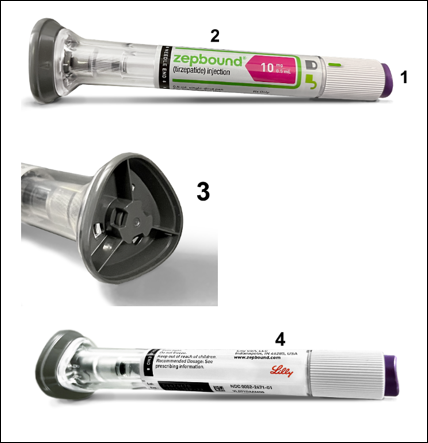 |
|
MOUNJARO® (TIRZEPATIDE) INDICATION AND SAFETY SUMMARY WITH WARNINGS
Mounjaro® (mown-JAHR-OH) is an injectable medicine for adults with type 2 diabetes used along with diet and exercise to improve blood sugar (glucose).
- It is not known if Mounjaro can be used in people who have had inflammation of the pancreas (pancreatitis). Mounjaro is not for use in people with type 1 diabetes. It is not known if Mounjaro is safe and effective for use in children under 18 years of age.
Warnings - Mounjaro may cause tumors in the thyroid, including thyroid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath. If you have any of these symptoms, tell your healthcare provider.
- Do not use Mounjaro if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC).
- Do not use Mounjaro if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Do not use Mounjaro if you are allergic to it or any of the ingredients in Mounjaro.
Mounjaro may cause serious side effects, including:
Inflammation of the pancreas (pancreatitis). Stop using Mounjaro and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Mounjaro with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include dizziness or light-headedness, sweating, confusion or drowsiness, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability, or mood changes, hunger, weakness and feeling jittery.
Serious allergic reactions. Stop using Mounjaro and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, and very rapid heartbeat.
Kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration.
Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Mounjaro. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
Changes in vision. Tell your healthcare provider if you have changes in vision during treatment with Mounjaro.
Gallbladder problems. Gallbladder problems have happened in some people who use Mounjaro. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), and clay-colored stools.
Common side effects
The most common side effects of Mounjaro include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, and stomach (abdominal) pain. These are not all the possible side effects of Mounjaro. Talk to your healthcare provider about any side effect that bothers you or doesn’t go away.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before using Mounjaro
- Your healthcare provider should show you how to use Mounjaro before you use it for the first time.
- Talk to your healthcare provider about low blood sugar and how to manage it.
- If you take birth control pills by mouth, talk to your healthcare provider before you use Mounjaro. Birth control pills may not work as well while using Mounjaro. Your healthcare provider may recommend another type of birth control for 4 weeks after you start Mounjaro and for 4 weeks after each increase in your dose of Mounjaro.
Review these questions with your healthcare provider:
❑ Do you have other medical conditions, including problems with your pancreas or kidneys, or severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems digesting food?
❑ Do you take other diabetes medicines, such as insulin or sulfonylureas?
❑ Do you have a history of diabetic retinopathy?
❑ Are you pregnant, plan to become pregnant, breastfeeding, or plan to breastfeed? It is not known if Mounjaro will harm your unborn baby or pass into your breast milk.
❑ Do you take any other prescription medicines or over-the-counter drugs, vitamins, or herbal supplements?
How to take
- Read the Instructions for Use that come with Mounjaro.
- Use Mounjaro exactly as your healthcare provider says.
- Mounjaro is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm.
- Use Mounjaro 1 time each week, at any time of the day.
- Do not mix insulin and Mounjaro together in the same injection.
- You may give an injection of Mounjaro and insulin in the same body area (such as your stomach area), but not right next to each other.
- Change (rotate) your injection site with each weekly injection. Do not use the same site for each injection.
- If you take too much Mounjaro, call your healthcare provider or seek medical advice promptly.
Learn more
Mounjaro is a prescription medicine. For more information, call 1-833-807-MJRO (833-807-6576) or go to www.mounjaro.lilly.com.
Please click to access the full Prescribing Information and Medication Guide for Mounjaro.
This summary provides basic information about Mounjaro but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your healthcare provider. Be sure to talk to your healthcare provider about Mounjaro and how to take it. Your healthcare provider is the best person to help you decide if Mounjaro is right for you.
TR CON CBS 14SEP2022
ZEPBOUND® (TIRZEPATIDE) INDICATION AND SAFETY SUMMARY WITH WARNINGS
Zepbound® (ZEHP-bownd) is an injectable prescription medicine that may help adults with obesity, or with excess weight (overweight) who also have weight-related medical problems, lose weight and keep it off. It should be used with a reduced-calorie diet and increased physical activity.
- Zepbound contains tirzepatide and should not be used with other tirzepatide-containing products or any GLP-1 receptor agonist medicines. It is not known if Zepbound is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products. It is not known if Zepbound can be used in people who have had pancreatitis. It is not known if Zepbound is safe and effective for use in children under 18 years of age.
Warnings - Zepbound may cause tumors in the thyroid, including thyroid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath. If you have any of these symptoms, tell your healthcare provider.
- Do not use Zepbound if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC).
- Do not use Zepbound if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Do not use Zepbound if you have had a serious allergic reaction to tirzepatide or any of the ingredients in Zepbound.
Zepbound may cause serious side effects, including:
Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Zepbound. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
Kidney problems (kidney failure). Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration.
Gallbladder problems. Gallbladder problems have happened in some people who use Zepbound. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), or clay-colored stools.
Inflammation of the pancreas (pancreatitis). Stop using Zepbound and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Serious allergic reactions. Stop using Zepbound and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, or very rapid heartbeat.
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Zepbound with medicines that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include dizziness or light-headedness, sweating, confusion or drowsiness, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability, mood changes, hunger, weakness or feeling jittery.
Changes in vision in patients with type 2 diabetes. Tell your healthcare provider if you have changes in vision during treatment with Zepbound.
Depression or thoughts of suicide. You should pay attention to changes in your mood, behaviors, feelings or thoughts. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you.
Common side effects
The most common side effects of Zepbound include nausea, diarrhea, vomiting, constipation, stomach (abdominal) pain, indigestion, injection site reactions, feeling tired, allergic reactions, belching, hair loss, and heartburn. These are not all the possible side effects of Zepbound. Talk to your healthcare provider about any side effect that bothers you or doesn’t go away.
Tell your healthcare provider if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before using Zepbound
- Your healthcare provider should show you how to use Zepbound before you use it for the first time.
- Tell your healthcare provider if you are taking medicines to treat diabetes including insulin or sulfonylureas which could increase your risk of low blood sugar. Talk to your healthcare provider about low blood sugar levels and how to manage them.
- If you take birth control pills by mouth, talk to your healthcare provider before you use Zepbound. Birth control pills may not work as well while using Zepbound. Your healthcare provider may recommend another type of birth control for 4 weeks after you start Zepbound and for 4 weeks after each increase in your dose of Zepbound.
Review these questions with your healthcare provider:
❑ Do you have other medical conditions, including problems with your pancreas or kidneys, or severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems digesting food?
❑ Do you take diabetes medicines, such as insulin or sulfonylureas?
❑ Do you have a history of diabetic retinopathy?
❑ Do you take any other prescription medicines or over-the-counter drugs, vitamins, or herbal supplements?
❑ Are you pregnant, plan to become pregnant, breastfeeding, or plan to breastfeed? Zepbound may harm your unborn baby. Tell your healthcare provider if you become pregnant while using Zepbound. It is not known if Zepbound passes into your breast milk. You should talk with your healthcare provider about the best way to feed your baby while using Zepbound.
- Pregnancy Exposure Registry: There will be a pregnancy exposure registry for women who have taken Zepbound during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry, or you may contact Lilly at 1-800-LillyRx (1-800-545-5979).
How to take
- Read the Instructions for Use that come with Zepbound.
- Use Zepbound exactly as your healthcare provider says.
- Zepbound is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm.
- Use Zepbound 1 time each week, at any time of the day.
- Change (rotate) your injection site with each weekly injection. Do not use the same site for each injection.
- If you take too much Zepbound, call your healthcare provider, seek medical advice promptly, or contact a Poison Center expert right away at 1-800-222-1222.
Learn more
Zepbound is a prescription medicine. For more information, call 1-800-LillyRx (1-800-545-5979) or go to www.zepbound.lilly.com.
Please click to access the full Prescribing Information and Medication Guide for Zepbound.
This summary provides basic information about Zepbound but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your healthcare provider. Be sure to talk to your healthcare provider about Zepbound and how to take it. Your healthcare provider is the best person to help you decide if Zepbound is right for you.
ZP CON CBS 08NOV2023
Mounjaro® and Zepbound® and their delivery device bases are registered trademarks owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.
About Lilly
Lilly is a medicine company turning science into healing to make life better for people around the world. We've been pioneering life-changing discoveries for nearly 150 years, and today our medicines help more than 51 million people across the globe. Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world's most significant health challenges: redefining diabetes care; treating obesity and curtailing its most devastating long-term effects; advancing the fight against Alzheimer's disease; providing solutions to some of the most debilitating immune system disorders; and transforming the most difficult-to-treat cancers into manageable diseases. With each step toward a healthier world, we're motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable. To learn more, visit Lilly.com and Lilly.com/news, or follow us on Facebook, Instagram and LinkedIn. P-LLY
Cautionary Statement Regarding Forward-Looking Statements
This open letter contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about the use of Mounjaro® (tirzepatide) and Zepbound® (tirzepatide) and reflects Lilly’s current beliefs and expectations. However, there can be no assurance that the use of Mounjaro® (tirzepatide) and Zepbound® (tirzepatide) will achieve Lilly’s objectives or that Lilly will execute its strategy as planned. For further discussion of risks and uncertainties relevant to Lilly’s business that could cause actual results to differ from Lilly’s expectations, see Lilly’s Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Lilly undertakes no duty to update forward-looking statements to reflect events after the date of this letter.
©Lilly USA, LLC 2024. All rights reserved.
